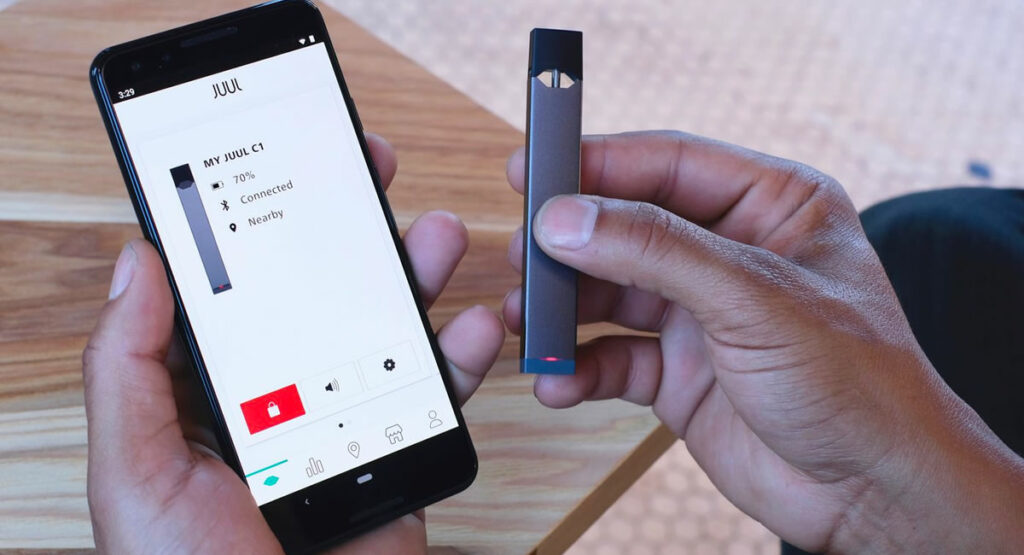
Juul has submitted an application to the FDA for a new “next generation” vape device aimed at preventing underage use. The proposed electronic cigarette integrates age verification technology and usage restrictions to combat youth vaping.
E-cigarette manufacturer Juul has faced intense scrutiny over its contribution to teenage nicotine addiction. In an effort to rehabilitate its image, Juul is proposing a high-tech vaporizer (juul2) equipped with age verification.
The device connects to an app that confirms users are adults through biometric scans and ID checks. It can also lock the device between puffs. Juul claims these innovations will prevent underage vaping and improve public health.
Will the advanced features be enough for FDA approval? This article reviews Juul’s redesigned vape and its potential impact.
Age Verification System
The primary addition to Juul’s new vaporizer is the age verification system. Users must complete a signup process on the accompanying smartphone app.
This involves uploading a photo ID to prove age. Biometric facial scanning matches selfies to the ID photo for authentication. Data is processed by a third party verifier.
Once validated as an adult, the Juul device unlocks for use. It can be locked again remotely through the app. This restricts unauthorized access and limits vaping to verified users.
The age verification blueprint stems from Juul’s system launched in 2021 in the UK. Applying advanced technology aims to make underage usage practically impossible.
Other Design Features
Along with age verification, Juul’s proposed vape includes various hardware changes:
- Bluetooth enabled for app connectivity
- Larger battery with LED indicator lights
- New heating element and pod improvements
- Tamper resistant pod construction
According to Juul, these updates enhance the overall performance and safety of the device. The changes cater toward adult smokers looking to switch from traditional cigarettes.
FDA Application Status
Juul’s premarket tobacco application for its new age-verified vape is currently under FDA review. The agency will evaluate the product’s risks and potential benefits to public health.
The FDA previously rejected an application for Juul’s existing vapes. However, a court appeal has temporarily prevented a sales ban. Juul hopes evidence around youth deterrence will sway FDA decision making this time.
Juul cites research showing vaping is less hazardous than smoking for adult cigarette users. The company aims to position its redesigned device as a harm reduction tool.
Reaction
Juul frames its proposed vape as a high-tech solution to preventing teen vaping. However, anti-tobacco groups remain skeptical of the company’s motives.
Critics point to Juul’s alleged role in fueling the youth vaping epidemic. Lawsuits continue over claims of deceptive marketing to adolescents. Juul maintains the new device demonstrates its commitment to keeping vapes away from kids.
The FDA approval process will thoroughly analyze the new age verification features. But some believe Juul is merely grasping for ways to remain on the market amidst regulatory pressure.
- UK Tobacco Ban 2026: The “Smokefree Generation” Law - March 4, 2026
- Myanmar Enacts Total Ban on E-Cigarettes and E-Shisha - February 25, 2026
- UK Announces Mandatory Vape Tax and Duty Stamps from 2027 - February 10, 2026